Do you know which vitamin supplements are best? Or do you know how to find out? It can be difficult to compare food supplements and find out which ones contain the most if you don't know the terms on the label. So here you get an explanation of the different terms on your food supplement label.
In Denmark, we have very clear rules for what information the packaging for food and nutritional supplements must contain. These rules are continuously regulated in line with new knowledge, new types of products and most recently in connection with an adaptation to European standardization.
What does the label say?
The labels on food supplements always contain 4 elements:
- Duty text
- Contents and ingredients
- Expiry date and Batch no.
- Information on the market leading company
What is duty text?
The mandatory text is fixed wording that must appear on all dietary supplements. It exists to ensure that the consumer uses and stores the product correctly. It is, for example, " keep out of the reach of small children ", or " food supplements should not replace a varied diet ".
What is the difference between Content and Ingredients?
Maybe you're thinking, well, the content and the ingredients aren't the same? Almost, but it must be listed in two different ways and contain different information.
The content , or more specifically the Nutrient content, is a table of the nutrients with a nutritional or physiological effect that the product contains. It can therefore be vitamins, minerals, herbs, proteins, amino acids, etc. Here the nutrients are listed with amount and RI% per daily dose.

The ingredient list, on the other hand, is a list of all the ingredients that the product contains. That is both nutrients, any fillers and binders/consistencies, colourings, preservatives and surface treatments. The ingredients are often a long list, where the ingredients must be listed in ranked order by largest quantity. Therefore, the ingredient the product contains the most of is listed first. In addition, allergens are written in bold to increase visibility.

Many may have already noticed that the vitamins and minerals do not always come first, but that the additives often do. This is because many nutrients do not take up much space, and therefore you have to add fillers to, for example, make a tablet.
What is the difference between mg, mcg, µg and IU?
When looking at a (nutrient) content table, the name of the nutrient, amount and RI% are listed. Depending on which vitamin or mineral it is, the amount will be given in either mg, mcg or µg. All units of measurement for weight and are thus:
- 1 mg means 1 milligram = 1/1,000 gram
- 1 mcg means 1 microgram = 1/1,000,000 gram
- 1 µg means 1 microgram = 1/1,000,000 gram
µ is the Greek letter “My”, and 1 mcg = 1 µg. There are therefore two different ways of writing micrograms.
In addition, IU (IE) is also used, which stands for International Unit. This unit of measurement is rarely used and most often in relation to vitamins A, E and D. For vitamin A, e.g. that 1 IU is 0.3 mcg of retinol or 0.6 mcg of beta-carotene, and the unit therefore depends on the vitamin source. It is therefore very difficult to compare and is actually illegal to use in Denmark.
What is reference intake and RI%?
The reference intake, abbreviated RI, is a value that should make it easier for consumers to compare the content of nutrients in different products. It is determined on the basis of a kind of average of an adult's daily intake of vitamins and minerals. RI% indicates how large a percentage of nutrients you get by taking the dietary supplement compared to what an average person eats and drinks in a day.
RI values must be used as a tool to compare the nutrient content of food supplements in relation to one's other daily diet.
The first averages were calculated by the United States during World War II, where they wanted to ensure that the soldiers who were sent to Europe avoided the same deficiency diseases that were experienced during World War I. Today, however, we know that people have very different needs for vitamins and minerals, as e.g. the elderly absorb nutrients less well, men, women, children and young people have different needs, and since athletes use more nutrients because they burn more and expose the body to stress. Just as well, one's diet means everything in terms of which vitamins and minerals you get daily.
RI is therefore NOT a recommendation or a measure for the amount of vitamins and minerals that must be consumed daily. RI is just a comparison tool for the consumer. For example, it is easy to assume that a product that contains 25% of the reference intake for vitamin C contains more vitamin C than a product that contains 15% of the reference intake. The RI values must therefore be used as a tool to compare the nutritional content of food supplements in relation to one's other daily diet.
What about the reference intake for children?
Since the need for vitamins and minerals is different for children and adults, Denmark has for many years had different RI values for children 1-10 years and adults. In January 2018, it was decided to follow the European standards, and therefore the RI values for children were removed. All nutritional supplements are therefore now based on the same reference values, which makes it easier to compare the content of nutritional supplements.
Which nutritional supplements are best?
When you are looking for food supplements, it is always a good idea to look at the contents of the product. If you lead, for example, after a multivitamin, it is a good idea to check which different vitamins and minerals the product contains. In addition, you must compare:
- The amounts of the various vitamins, e.g. by looking at RI%
- The recommended daily dose
- The number of tablets/capsules in the box
- The price of the can
- The size of the capsule/tablet, if you e.g. have difficulty swallowing tablets
As a starting point, you would like to have a maximum of content per tablet/capsule and the most tablets/capsules in the can in relation to the price.
An example could be:
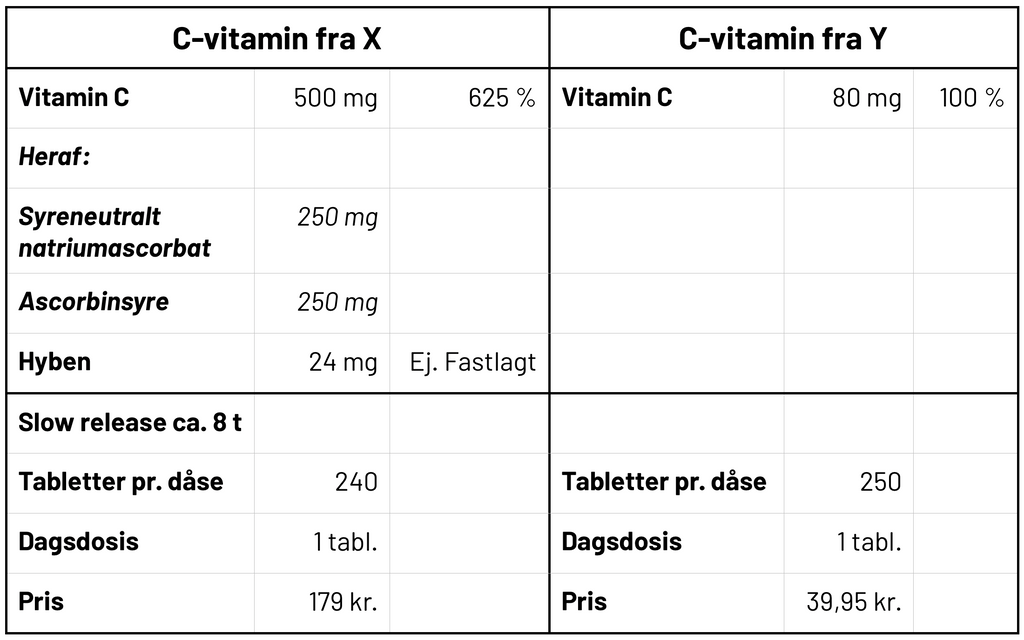
Here we have two vitamin C supplements. When you look at them, someone might think - I take vitamin C from Y, as it is the cheapest and contains the most tablets. But if you look more closely at the contents, you quickly see that vitamin C from X contains 500 mg per tablet, where Y's only contains 80 mg per tablet.
As you can see from the RI%, you get 6.25 times more vitamin C per tablet with X's vitamin C. In addition, X's vitamin C has a slow-release effect, which means that the tablet slowly dissolves and releases vitamin C over approx. 8 hours. Therefore, the body can better absorb the vitamin C and the body has the vitamin when it needs it - e.g. when you strength train or go for a run. Less is thus wasted and is excreted with the urine.
Go for the product with the most nutrients and the most content per tablet, as large cans with many tablets are not always the best.
If you were to achieve the same amount and effect with Y's vitamin C, you would have to eat 6.25 tablets spread over the whole day. In terms of price, it would therefore be more expensive to eat vitamin C from Y, since you have to buy 6 glasses to get the same amount of vitamin C as 1 can from X then: 240 tablets x 6.25 / 250 tablets per glasses = 6 glasses. This gives a total price of DKK 239.70.
Here it was relatively easy to compare the above products, as they only contain one vitamin.
Should you e.g. comparing a multivitamin immediately becomes difficult. Here my recommendation is that you look at the number of different vitamins, minerals and other nutrients the multivitamin contains, and go for the one with the most nutrients and content per serving. tablet, as large cans with many tablets are not always the best.
Sources:
The Danish Veterinary and Food Administration: https://altomkost.dk/fakta/kosttilskud/maerkning-af-kosttilskud/
Health And Science: https://www.healthandscience.eu/index.php?option=com_content&view=article&id=640:er-referenceintag-ri-og-anbefalet-daglig-filforsel-adt-bassent-pa-varm-luft&catid= 20&Itemid=269&lang=da
European Parliament and Council Regulation (EU) No. 1186/2011
